Developmental biology
From Wikipedia, the free encyclopedia
"Developmental genetics" redirects here. For the journal formerly known as Developmental Genetics, see Genesis (journal).
For the journal, see Developmental Biology (journal).
| This article needs additional citations for verification. (July 2007) (Learn how and when to remove this template message) |
Views of a Fetus in the Womb, Leonardo da Vinci, c. 1510 - 1512. The subject of prenatal development is a major subset of developmental biology.
Contents
Perspectives
The main processes involved in the embryonic development of animals are: regional specification, morphogenesis, cell differentiation, growth, and the overall control of timing. Regional specification refers to the processes that create spatial pattern in a ball or sheet of initially similar cells. This generally involves the action of cytoplasmic determinants, located within parts of the fertilized egg, and of inductive signals emitted from signaling centers in the embryo. The early stages of regional specification do not generate functional differentiated cells, but cell populations committed to develop to a specific region or part of the organism. These are defined by the expression of specific combinations of transcription factors. Morphogenesis relates to the formation of three-dimensional shape. It mainly involves the orchestrated movements of cell sheets and of individual cells. Morphogenesis is important for creating the three germ layers of the early embryo (ectoderm, mesoderm and endoderm) and for building up complex structures during organ development. Cell differentiation relates specifically to the formation of functional cell types such as nerve, muscle, secretory epithelia etc. Differentiated cells contain large amounts of specific proteins associated with the cell function. Growth involves both an overall increase in size, and also the differential growth of parts (allometry) which contributes to morphogenesis. Growth mostly occurs through cell division but also through changes of cell size and the deposition of extracellular materials. The control of timing of events and the integration of the various processes with one another is the least well understood area of the subject. It remains unclear whether animal embryos contain a master clock mechanism or not. The development of plants involves similar processes to that of animals. However plant cells are mostly immotile so morphogenesis is achieved by differential growth, without cell movements. Also, the inductive signals and the genes involved in plant development are different from those that control animal development.Developmental model organisms
Much of developmental biology research in recent decades has focused on the use of a small number of model organisms. It has turned out that there is much conservation of developmental mechanisms across the animal kingdom. In early development different vertebrate species all use essentially the same inductive signals and the same genes encoding regional identity. Even invertebrates use a similar repertoire of signals and genes although the body parts formed are significantly different. Model organisms each have some particular experimental advantages which have enabled them to become popular among researchers. In one sense they are "models" for the whole animal kingdom, and in another sense they are "models" for human development, which is difficult to study directly for both ethical and practical reasons. Model organisms have been most useful for elucidating the broad nature of developmental mechanisms. The more detail is sought, the more they differ from each other and from humans.Vertebrates:
- Frog: Xenopus (X.laevis and tropicalis).[1][2] Good embryo supply. Especially suitable for microsurgery.
- Zebrafish: Danio rerio.[3] Good embryo supply. Well developed genetics.
- Chicken: Gallus gallus.[4] Early stages similar to mammal, but microsurgery easier. Low cost.
- Mouse: Mus musculus.[5] A mammal with well developed genetics.
- Fruit fly: Drosophila melanogaster.[6] Good embryo supply. Well developed genetics.
- Nematode: Caenorhabditis elegans.[7] Good embryo supply. Well developed genetics. Low cost.
Also popular for some purposes have been sea urchins[8] and ascidians.[9] For studies of regeneration urodele amphibians such as the axolotl Ambystoma mexicanum are used,[10] and also planarian worms such as Schmidtea mediterranea.[11] Plant development has focused on the thale cress Arabidopsis thaliana as a model organism.[12]
Developmental processes
Embryonic development of animals
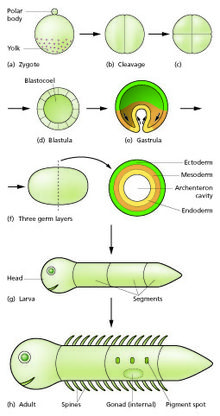
Generalized scheme of embryonic development. Slack "Essential Developmental Biology" Fig.2.8
The initial stages of human embryogenesis.
Regional specification is initiated by the presence of cytoplasmic determinants in one part of the zygote. The cells that contain the determinant become a signaling center and emit an inducing factor. Because the inducing factor is produced in one place, diffuses away, and decays, it forms a concentration gradient, high near the source cells and low further away.[16][17] The remaining cells of the embryo, which do not contain the determinant, are competent to respond to different concentrations by upregulating specific developmental control genes. This results in a series of zones becoming set up, arranged at progressively greater distance from the signaling center. In each zone a different combination of developmental control genes is upregulated.[18] These genes encode transcription factors which upregulate new combinations of gene activity in each region. Among other functions, these transcription factors control expression of genes conferring specific adhesive and motility properties on the cells in which they are active. Because of these different morphogenetic properties, the cells of each germ layer move to form sheets such that the ectoderm ends up on the outside, mesoderm in the middle, and endoderm on the inside.[19][20] Morphogenetic movements not only change the shape and structure of the embryo, but by bringing cell sheets into new spatial relationships they also make possible new phases of signaling and response between them.
Growth in embryos is mostly autonomous.[21] For each territory of cells the growth rate is controlled by the combination of genes that are active. Free living embryos do not grow in mass as they have no external food supply. But embryos fed by a placenta or extraembryonic yolk supply can grow very fast, and changes to relative growth rate between parts in these organisms help to produce the final overall anatomy.
The whole process needs to be coordinated in time and how this is controlled is not understood. There may be a master clock able to communicate with all parts of the embryo that controls the course of events, or timing may depend simply on local causal sequences of events.[22]
Cell differentiation
The Notch-delta system in neurogenesis.(Slack Essential Dev Biol Fig 14.12a)
Cell differentiation is usually the final stage of development, preceded by several states of commitment which are not visibly differentiated. A single tissue, formed from a single type of progenitor cell or stem cell, often consists of several differentiated cell types. Control of their formation involves a process of lateral inhibition,[25] based on the properties of the Notch-Delta signaling system.[26] For example, in the neural plate of the embryo this system operates to generate a population of neuronal precursor cells in which NeuroD is highly expressed.
Regeneration
Regeneration indicates the ability to regrow a missing part.[27] This is very prevalent amongst plants, which show continuous growth, and also among colonial animals such as hydroids and ascidians. But most interest by developmental biologists has been shown in the regeneration of parts in free living animals. In particular four models have been the subject of much investigation. Two of these have the ability to regenerate whole bodies: Hydra, which can regenerate any part of the polyp from a small fragment,[28] and planarian worms, which can usually regenerate both heads and tails.[29] Both of these examples have continuous cell turnover fed by stem cells and, at least in planaria, at least some of the stem cells have been shown to be pluripotent.[30] The other two models show only distal regeneration of appendages. These are the insect appendages, usually the legs of hemimetabolous insects such as the cricket,[31] and the limbs of urodele amphibians.[32] Considerable information is now available about amphibian limb regeneration and it is known that each cell type regenerates itself, except for connective tissues where there is considerable interconversion between cartilage, dermis and tendons. In terms of the pattern of structures, this is controlled by a re-activation of signals active in the embryo. There is still debate about the old question of whether regeneration is a "pristine" or an "adaptive" property.[33] If the former is the case, with improved knowledge, we might expect to be able to improve regenerative ability in humans. If the latter, then each instance of regeneration is presumed to have arisen by natural selection in circumstances particular to the species, so no general rules would be expected.Metamorphosis
Developmental processes are very evident during the process of metamorphosis. This occurs in various types of animal. Well known are the examples of the frog, which usually hatches as a tadpole and metamorphoses to an adult frog, and certain insects which hatch as a larva and then become remodeled to the adult form during a pupal stage.All the developmental processes listed above occur during metamorphosis. Examples that have been especially well studied include tail loss and other changes in the tadpole of the frog Xenopus,[34][35] and the biology of the imaginal discs, which generate the adult body parts of the fly Drosophila melanogaster.[36][37]